Which Ions Have the Same Electron Configuration as Argon
Na and CI d. Answer C a 2 K C l and P 3 View Answer Discussion You must be signed in to discuss.
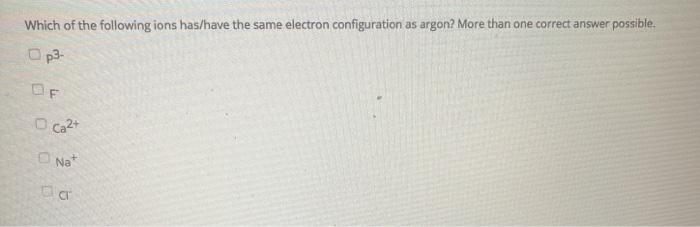
Solved Which Of The Following Ions Has Have The Same Chegg Com
Sodium-ion N a has the same electron configuration as a neon atom.
. Ca2 o Nat Which of the following explanations invoking the atom effect explains why H2S is a stronger acid than H20. More than one correct answer possible. The electron configuration of argon is 1s22s22p63s23p6.
What noble gas has the same electron configuration an K1 plus. -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds.
This means it has 17 electronsIt gains one electron to give. Argon Ar Potassium K Calcium Ca Chromium Cr Cr 2 Cr 3 Copper Cu Cu Cu 2 Iron Fe Fe 2 Fe 3 Read my article in Science Education based on my dissertation. It wants you to list for ions that have the same number of electrons as are gone.
Argon is the third-most abundant gas in the Earths atmosphere at 0934 9340 ppmv. The chemical symbol for Argon is Ar. Calcium reacts with fluorine to form.
Chlorine Cl has atomic number of 17. This means it has 19 electronsIt lose one electron to give. Which of the following ions hashave the same electron configuration as argon.
Electron configuration of a fluoride ion F- is. The electron configuration of chlorine is 1s22s22p63s23p5. The argon atom Ar which precedes potassium in the periodic table of elements has the same configuration 1s2 2s2 2p6 3s2 3p6 as the potassium ion K with 18 electrons.
S-2 and P-3 c. Watch More Solved Questions in Chapter 4 Problem 1 Problem 2 Problem 3 Problem 4 Problem 5 Problem 6 Problem 7 Problem 8 Problem 9 Problem 10 Problem 11 Problem 12 Problem 13 Problem 14. Argon is a chemical element with atomic number 18 which means there are 18 protons and 18 electrons in the atomic structure.
Which of the following ions will have the same electron orbital configuration as the argon atom. List four ions that have the same number of electrons as argon Ar. Which of the following ions has the same electron configuration as an argon atom.
Both are isoelectronic species with 10 electrons and the electronic configuration 1 s 2 2 s 2 2 p 6 Sodium has electronic configuration 1 s 2 2 s 2 2 p 6 3 s 1. The electronic configuration is. In addition the calcium ion with a charge of 2 and the scandium ion with a charge of 3 also have the same electronic configuration as potassium ion K with 18 electrons.
Potassium K has atomic number of 19. It is mainly due to the greater electronegativity of sulfur. All the elements in a particular group of the periodic table have the same number of.
K and argon have the same electron configuration. Helium has two electrons in the valence shell argon has also the same condition it have eight electrons in valence shell so the higher amount of energy required to remove the electrons so these not form the ions. We see that it has a total of 18 electrons where the last six electrons air placed in the three p orbital with 18 electrons we can then go to something like sulfur which in this case on Lee has 16 electrons because it only.
Argon and the chloride ion have the same electron configuration. To do this it might be helpful to first right out the electron configuration of argon. Magnesium reacts with sulfur to form.
Argon has an atomic number of 18.

Electron Configurations Ck 12 Foundation

See The Electron Configuration Diagrams For Atoms Of The Elements Potassium Atom Atom Diagram Electron Configuration

Electron Configurations Ck 12 Foundation

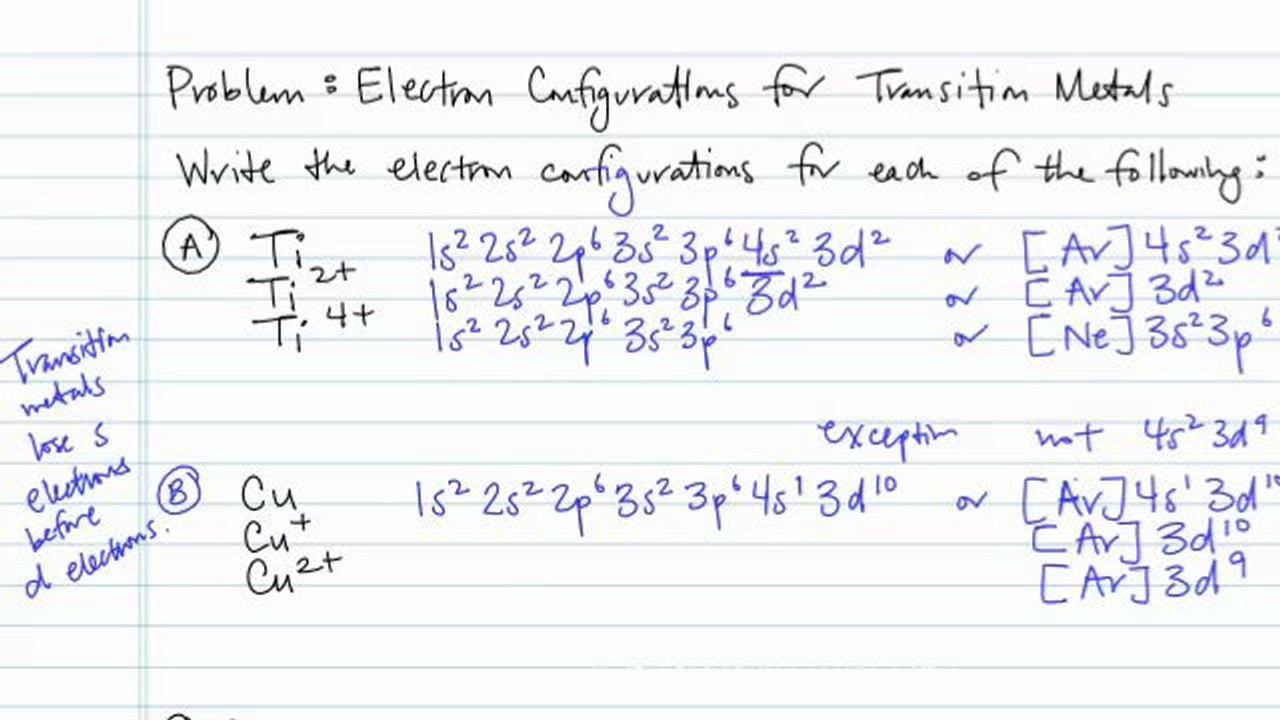
Electron Configurations For Transition Metals And Their Ions Problem Concept Chemistry Video By Brightstorm
Comments
Post a Comment